[最も選択された] heterogeneous and homogeneous equilibrium 318218-State homogeneous and heterogeneous equilibrium
Homogeneous and Heterogeneous Equilibrium Video Lecture from Chemical equilibrium Chapter of Chemistry Class 11 for HSC, IIT JEE, CBSE & NEETWatch PreviousHomogeneous and heterogeneous equilibrium is a system of chemical equilibrium which depend upon the states of matter of the substances involved Homogeneous equilibrium involves substances in the same state Heterogeneous equilibrium involves substances in different statesHeterogeneous solutionA solution composed of different states of matter equilibriumThe state of a reaction in which the rates of the forward and reverse reactions are the same homogeneous solutionA solution composed of matter that all exists in the same state

Introduction To Heterogeneous Chemical Equilibrium Youtube
State homogeneous and heterogeneous equilibrium
State homogeneous and heterogeneous equilibrium-Heterogeneous Agents and General Equilibrium in Financial Markets Tyler Abbot Abstract This paper investigates the e ects of heterogeneous preferences on risk premia, on asset price dynamics, and on distributions of wealth and consumption I consider aIn heterogeneous equilibrium, substances are in different phases Key Terms equilibrium The state of a reaction in which the rates of the forward and reverse reactions are the same heterogeneous solution A solution composed of different states of matter homogeneous solution A solution composed of matter that all exists in the same state




6 2 Equilibrium Constants 1718 Chemical Equilibrium Dissociation Chemistry
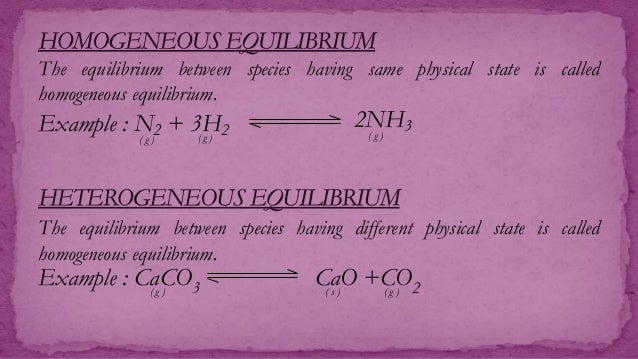
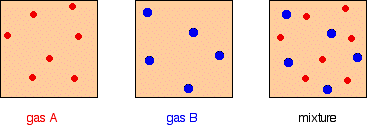
Homogeneous equilibrium ( plural homogeneous equilibria ) ( chemistry) An equilibrium all participating substances of which are in the same phase quotations 1995, Ken Gadd, Advanced GNVQ Science, page 261 The equilibrium is called a homogeneous equilibrium if all of the reactants and products are in the same phase, that is, all gases, allHeterogeneous system has at least two phases a mixture of solids or immiscible liquids constitutes a heterogeneous system Any system consisting of two or more phases is called heterogeneous system Homogeneous and Heterogeneous Equilibrium Systems Equilibrium can be established in either type of systems The key difference between homogeneous and heterogeneous equilibrium is that in homogeneous equilibrium, the reactants and products are in the same phase of matter whereas, in heterogeneous equilibrium, the reactants and products are in different phases Equilibrium is a state in which the concentrations of reactants and products remain constant There are two types of equilibria
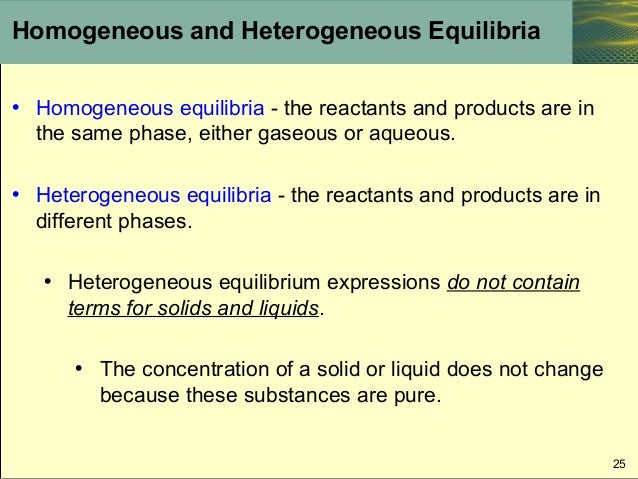
Homogeneous Equilibria A homogeneous equilibrium is one in which all of the reactants and products are present in a single solution (by definition, a homogeneous mixture) In this tutorial, we will concentrate on the two most common types of homogeneous equilibria those occurring in liquidphase solutions and those involving exclusively gaseous speciesHeterogeneous Equilibria A homogeneous equilibrium occurs when all reagents and products are found in the same phase (solid, liquid, or gas) and a heterogeneous equilibrium is when they are in different phases The position of heterogeneous equilibrium does not depend on the amount of pure solid and liquid presentHeterogeneous Equilibria A heterogeneous equilibrium is a system in which reactants and products are found in two or more phases The phases may be any combination of solid, liquid, or gas phases, and solutions When dealing with these equilibria, remember that solids and pure liquids do not appear in equilibrium constant expressions (the activities of pure solids, pure
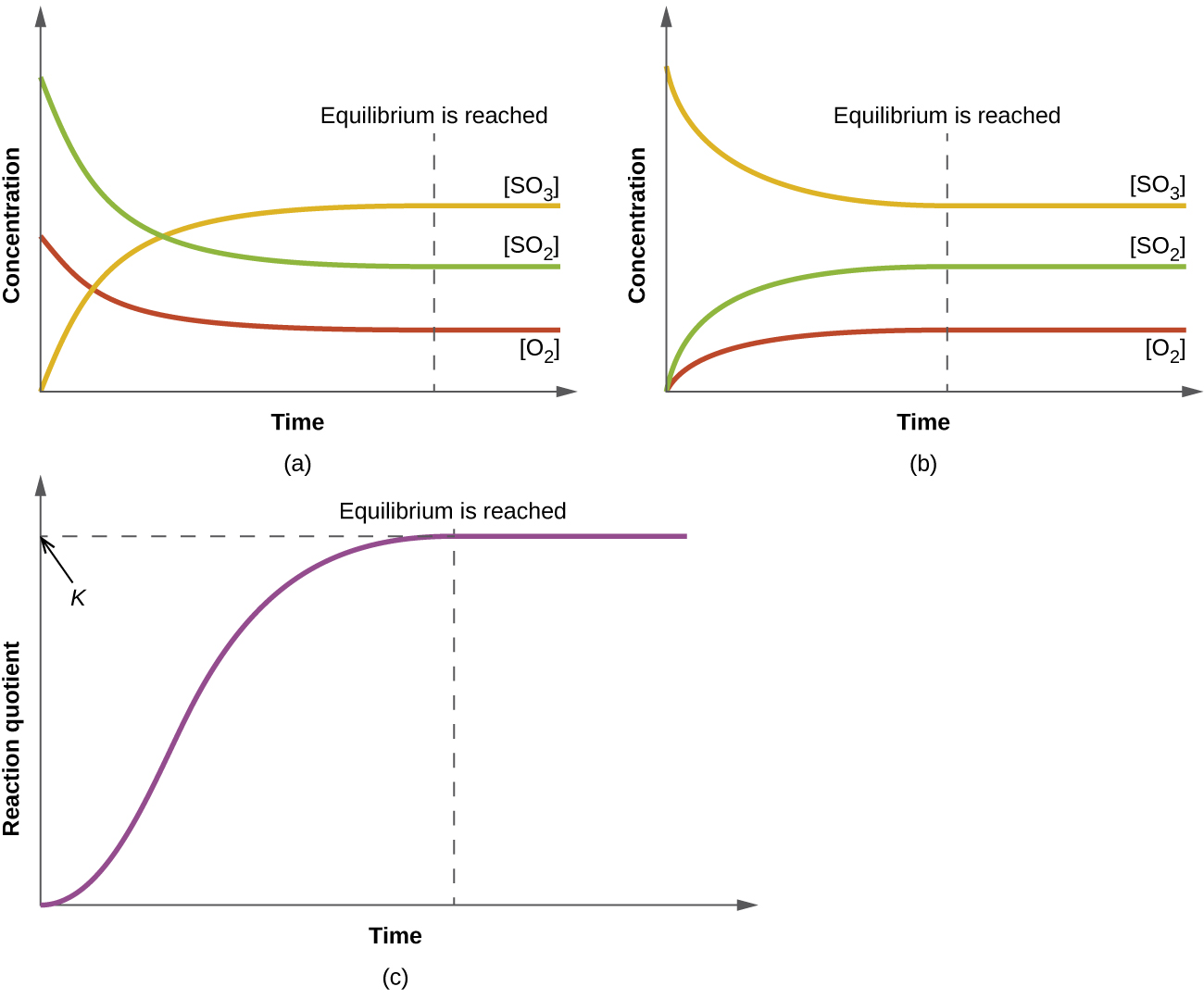
Watch more videos on http//wwwbrightstormcom/science/chemistrySUBSCRIBE FOR All OUR VIDEOS!https//wwwyoutubecom/subscription_center?add_user=brightstorA homogeneous equilibrium is an equilibrium reaction in which all the reactants and products are in the same phase Example 1==> 2SO2(g)O2(g)>2SO3 (g) 2==>N2(g)3H2(g)————> 2NH3(g) Inboth gases all the reactants and products are iIn keeping with the hetero and homo prefix theme, it should be no surprise that a homogenous equilibrium reaction is a reaction where all of the products and reactants are in




Equilibrium Notes Chem1100 Chemistry 1 Studocu




Homogeneous Gaseous Equilibria Ppt Download
Re Homogeneous vs Heterogeneous Equilibria Post by Samantha Miceli 3J » Sun 1031 pm To the best of my understanding, a phase is the form of matter (solid, liquid, gas), so "in the same phase" means that the reactants andHomogeneous equilibrium Heterogeneous equilibrium 'Homo' and 'hetero' are the prefixes which are originated from Greek words for 'similar' and 'different' respectively As the name suggests heterogeneous equilibrium is that equilibrium system in which reactants and products are found in two or more states of matterWhat is the difference between homogeneous equilibrium and heterogeneous equilibrium?




Class 11 Equilibrium Homogeneous And Heterogeneous Equilibria



1
Welcome to Sarthaks eConnect A unique platform where students can interact with teachers/experts/students to get solutions toHomogenous and heterogeneous equilibrium Expression for the equilibrium constant of a reaction is written in the form of a ratio The numerator consists of the molar concentration (or partial pressure) terms of the products each raised to a power equal to its stoichiometric coefficient in the balanced chemical equation, and the denominatorHomogenous Equilibria A homogeneous equilibrium is one in which all of the reactants and products are present in a single solution (by definition, a homogeneous mixture) Reactions between solutes in liquid solutions belong to one type of homogeneous equilibria




6 2 Equilibrium Constants 1718 Chemical Equilibrium Dissociation Chemistry




Chemical Equilibrium Concept Of Dynamic Equilibrium Heterogeneous Equilibrium
Equilibrium Law for Heterogeneous Reactions In heterogeneous systems where two or more phases are present at equilibrium (eg solid and gas), the equilibrium is unaffected by the amounts of pure solids or pure liquids present, as long as some of each is present This is because the concentrations of solids and liquids are constant(b) there exist purestrategy equilibria in which the right number but the wrong set of individuals get vaccinated when c D ∈ (p {j} i, p ∅ i;In the history of thermodynamics, On the Equilibrium of Heterogeneous Substances is a 300page paper written by American chemical physicist Willard GibbsIt is one of the founding papers in thermodynamics, along with German physicist Hermann von Helmholtz's 18 paper "Thermodynamik chemischer Vorgänge" Together they form the foundation of chemical




Comparison Between Homogeneous And Heterogeneous Treatments This Download Scientific Diagram



Exe
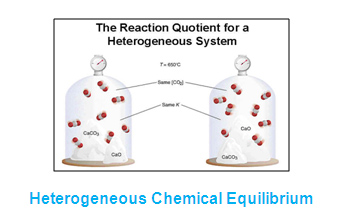
A homogeneous equilibrium occurs when all reagents and products are found in the same phase (solid, liquid, or gas) and a heterogeneous equilibrium is when they are in different phases To demonstrate a heterogeneous equilibrium, we'll discuss the equilibrium expression for when calcium carbonate dissolves in water (a process known as In a heterogeneous reaction (where the states are varied) we do not include liquids and solids in the equilibrium equation because their concentrations do not change Eg Chemguidecouk However, when it is a homogenous equation we DO include solids and liquidsHeterogeneous Equilibrium If the reactants and products of a reaction in equilibrium, are in different phases, then it is called as heterogeneous equilibrium Example H 2 O(l) ⇄ H 2 O(g) CaCO 3 (s) ⇄ CaO(s) CO 2 (g) A homogeneous equilibrium is one in which all species are present in the same phase Common examples include gasphase or




Chemical Equilibrium Accessscience From Mcgraw Hill Education




Analysis Of Soil Slope Stability In Heterogeneous And Homogeneous Soil Under Leakage Based On Numerical And Limit Equilibrium Methods Semantic Scholar
Class 11 Equilibrium Homogeneous and Heterogeneous Equilibria 11th Chemistry Equilibrium Homogeneous and Heterogeneous EquilibriaReaction Equilibrium I Heterogenous vs Homogenous Reactions A Homogeneous reaction – all reactants and products are in one phase • • • All in the gaseous phase, or All in solution (aqueous) H 2 O (g) CO (g) H 2 (g) CO 2 (g) B Heterogeneous reaction – reactants in two phases • Zn(s) 2 HCl(aq) H 2(g) ZnWhen the state of equilibrium in a system has components in more than one phase it is termed as a heterogeneous equilibrium For example, if we take a container with ice and water at a temperature that is allowing the existence of both the phases simultaneously, such that, both ice and water are present in a state of equilibrium




Chemical Systems Involving Two Competitive Self Catalytic Reactions Abstract Europe Pmc




Equilibrium Kc Vs Kp Villanova College Chemistry Blog
Homogeneous and heterogeneous equilibrium is a system of chemical equilibrium which depend upon the states of matter of the substances involved Homogeneous equilibrium involves substances in the same state Heterogeneous equilibrium involves substances in different states An equilibrated system that contains products and reactants in a single phase is a homogeneous equilibrium; Explain homogeneous and heterogeneous equilibrium giving examples asked in Chemistry by jisu zahaan (297k points) cbse;



Eage Tutor



Chui Science Weebly Com Uploads 1 3 4 6 Problem Set 2 Solutions Pdf
The discussed models range from ideal monocomponent sorption on homogeneous (Langmuir) and heterogeneous sites, to multicomponent ideal sorption on homogeneous and heterogeneous sites, multicomponent multisite ion complexation with charge distribution (CDMUSIC) and nonideal competitive adsorption on heterogeneous sites (NICA) If all the reactants and products present in an equilibrium mixture are in same phase → homogeneous equilibrium Example N 2(g) 3H 2(g) ⇌ 2NH 3(g) If all the reactants and products present in an equilibrium mixture are indifferent phase → heterogeneous equilibrium Example CaCO 3 (s) ⇌ CaO (s) CO 2(g)In order to simplify the problems and understand the concept, we divide such reactions into different categories, namely, the homogeneous reactions, where the components involved in the reaction are present in the same phase and the heterogeneous reactions, where the components involved are present in different phases




Homogenous Vs Heterogenous Equilibrium Chemical Equilibrium Class 9 Chemistry 11th Chemistry Equilibrium



Equilibrium
In heterogeneous equilibrium, substances are in different phases What is the difference between homogeneous and heterogeneous examples?When calculating equilibrium constants, pure solids and liquids are not taken into account This is because their concentration does not change during the reaction In homogeneous equilibrium, all substances are in the same phase The key difference between homogeneous and heterogeneous equilibrium is that in homogeneous equilibrium, the reactants and products are in the same phase of matter whereas, in heterogeneous equilibrium, the reactants and products are in different phases Equilibrium is a state in which the concentrations of reactants and products remain constant




Homogeneous And Heterogeneous Equilibria




Homogeneous Vs Heterogeneous Equilibrium Reactions Study Com
Homogeneous or heterogeneous How would you separate mixtures? A homogeneous equilibrium is one in which all species are present in the same phase Common examples include gasphase or solution reactions A heterogeneous equilibrium is one in which species exist in more than one phase Common examples include reactions involving solids and gases, or solids and liquidsHomogeneous equilibria (a) and (b) Heterogeneous equilibria (c) and (d) (i) The initial effect on the vapour pressure of increasing the volume of the container will be



Chemical Equilibrium




Homogeneous And Heterogeneous Equilibria
Determination of the equilibrium constants The equilibrium constants in homogeneous and heterogeneous systems of ketoconazole were determined at 250 01 ºC and constant ionic strength of 01 M (NaCl) Potentiometric determination of K a1 mL of solution containing 1 103 M of ketoconazoleThe nozzle model utilised the delayed equilibrium model with two distinct nucleation approaches solely homogenous nucleation and a superposition of homogeneous and heterogeneous nucleation TheHomogeneous Mixtures Heterogeneous Mixtures centrifugation coagulation distillation evaporation filtration hand picking magnetic separation sieving winnowing sedimentation Mixture Separation Techniques True solution




Topic 6 Equilibria Chemistrycorner



Chemical Equilibrium Reaction Definition Examples Types
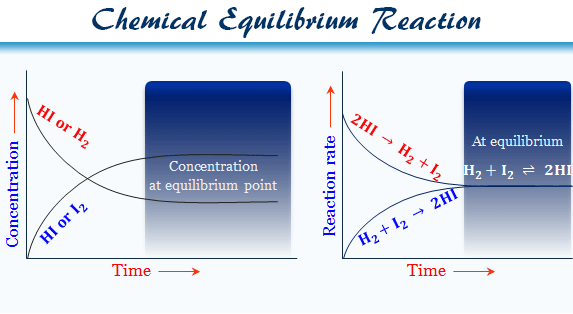
A system whose reactants, products, or both are in more than one phase is a heterogeneous equilibriumA homogeneous solution tends to be identical, no matter how you sample it Homogeneous mixtures are sources of water, saline solution, someHomogeneous and heterogeneous equilibria Homogeneous equilibrium In a homogeneous equilibrium, all the reactants and products are in the same phase For example H 2 (g) I 2 (g) ⇌ 2HI (g) In the above equilibrium, H 2, I 2 and HI are in the gaseous state Similarly, for the following reaction, all the reactants and products are in homogeneous solution phase Heterogeneous equilibrium
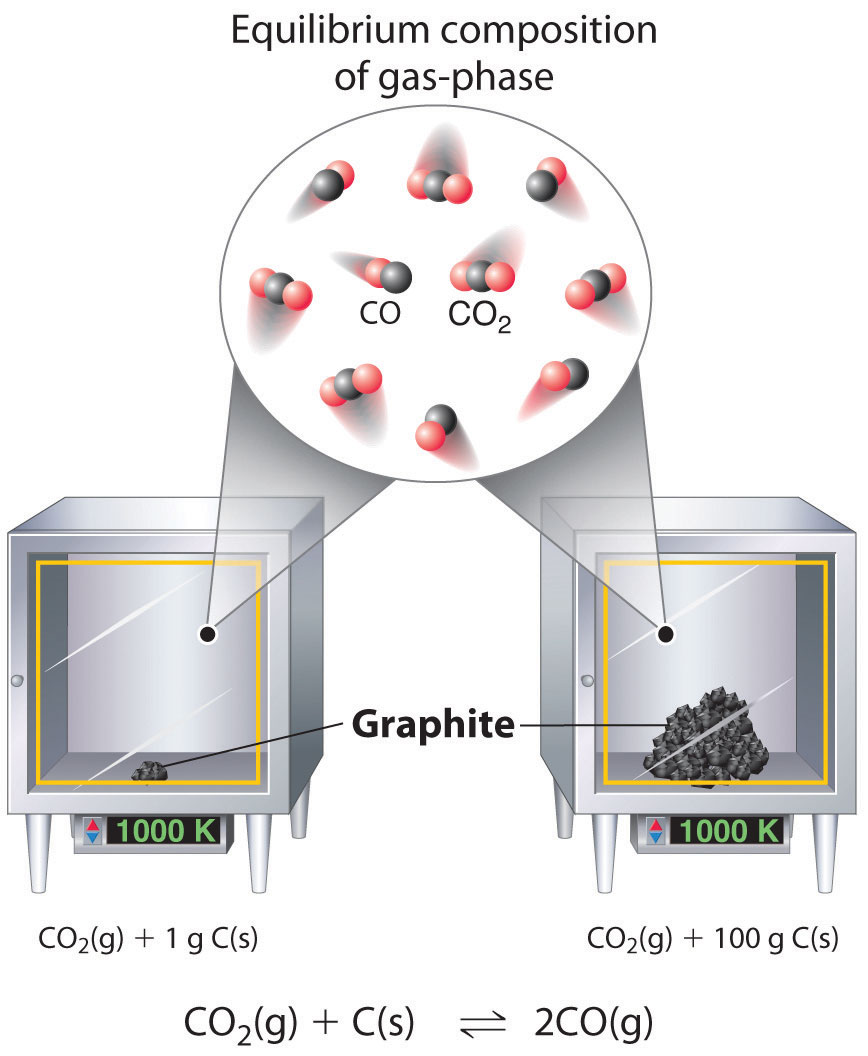



15 4 Heterogeneous Equilibria Chemistry Libretexts




Heterogeneous Equilibria Calculation of equilibrium concentrations or partial pressures for heterogeneous equilibria is very similar to the process for homogeneous equilibria (already discussed) The essential difference is that the activity for solids and pure liquids in the equilibrium constant expression is always equal to "1" 4 In a 3person homogeneous star network, (a) there exists a purestrategy equilibrium in which too few individuals get vaccinated when c D ∈ (p ∅ 1, p ∅ 1 2 (p ∅ i − β);A system whose reactants, products, or both are in more than one phase is a heterogeneous equilibrium




Which Of The Following Represents A Heterogeneous Equ




What Is Homogeneous And Heterogeneous Equilibrium Give Examples
A heterogeneous equilibrium has things present in more than one phase The usual examples include reactions involving solids and gases, or solids and liquids K c in homogeneous equilibria This is the more straightforward case It applies where everything in the equilibrium mixture is present as a gas, or everything is present in the same An equilibrated system that contains products and reactants in a single phase is a homogeneous equilibrium;Equilibrium is a state of chemical reaction where the rate of forward and backward reaction is the same Moreover, an equilibrium can be of two types homogeneous equilibrium and heterogeneous equilibrium A homogeneous equilibrium is defined as a homogeneous mixture (reactants and products in a single solution) in one phase




Solved Compare Homogeneous And Heterogeneous Equilibria




For Homogeneous Equilibrium Why Are Liquids And Solids Included In The Equilibrium Constant When They Aren T In Heterogeneous Equilibria Chemistry Stack Exchange
A homogeneous equilibrium is where all the reactants and products are present in the same physical state ie all the reactants and products are either in liquid or all of them are in the gaseous state Here in option A, all the reactant and products are present in the same stateB) heterogeneous chemical equilibrium c) neither homogeneous nor heterogeneous d) both homogeneous and heterogeneous Answer a Clarification In homogeneous equilibrium, the reactants and products are present in the same phase or physical state Nitrogen, Oxygen, and nitrogen monoxide are present in a gaseous state, so it is homogeneous
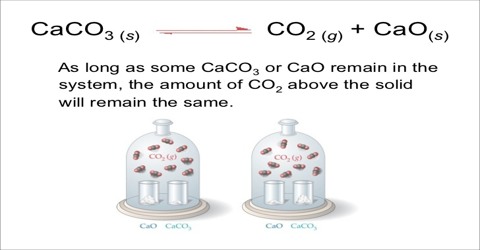



Heterogeneous Equilibria Qs Study




Homogeneous Vs Heterogeneous Equilibrium Reactions Study Com



Chemical Equilibrium Wikipedia



Www Sd308 Org Cms Lib Il Centricity Domain 1456 Uw equilibrium ws key Pdf




Equilibrium Resources




Equilibrium Acids And Bases Ppt Download




Energy Aspects Of Homogeneous Heterogeneous Transition In Formation Of Download Scientific Diagram




Class11 Homogeneous Equilibrium And Heterogeneous Equilibrium With Examples Explanations In Telugu Youtube
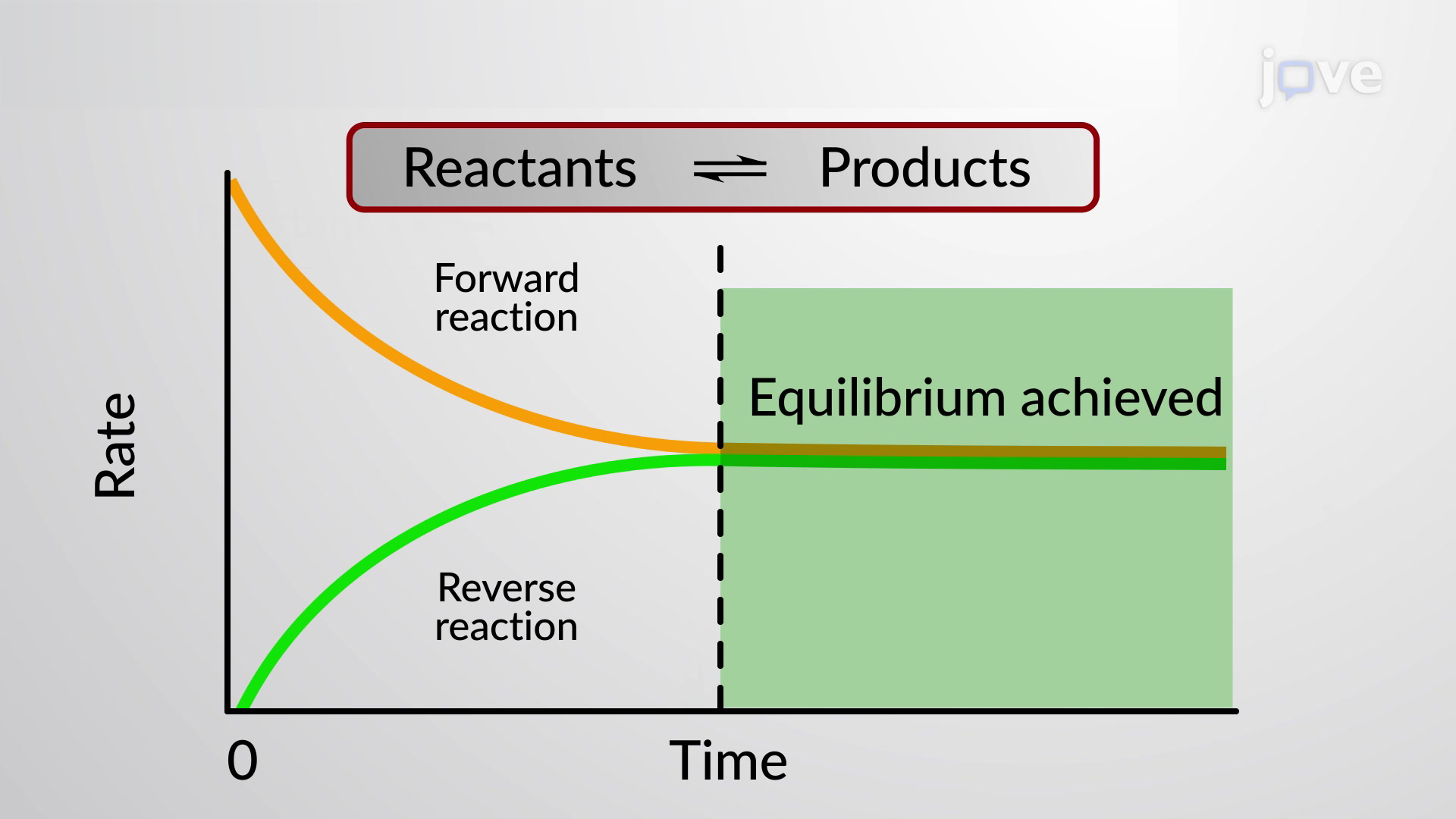



Jove Science Education Chemical Equilibrium




U U T V V Are Heterogeneous Systems 1v Vare Homogeneous I Ii Iii Are Heterogeneous




Homogeneous Equilibria Youtube




The Position Of Equilibrium Topic 7 2 Equilibrium




Equilibrium Gen Chem 10 Libguides At North Carolina Central University




Topics In Chm 1046 Intermolecular Forces Imf Themodynamics Ppt Download




Equilibrium Law And The Equilibrium Constant Section 7




Equilibrium Performance Of Homogeneous And Heterogeneous Agent Download Scientific Diagram




Introduction To Heterogeneous Chemical Equilibrium Youtube



1



Chm 1046



The Heterogeneous Equilibrium



Plos One Dynamical Selection Of Nash Equilibria Using Reinforcement Learning Emergence Of Heterogeneous Mixed Equilibria




Homogeneous Vs Heterogeneous Equilibrium Reactions Study Com




Homogeneous And Heterogeneous Equilibrium Chemical Equilibrium Chemistry Class 11 Youtube



Exe
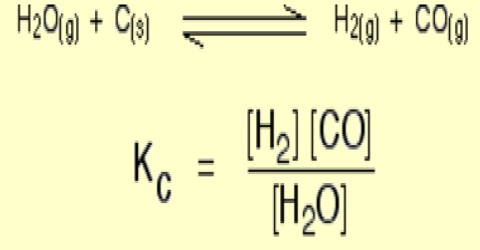



Homogeneous Equilibria Qs Study




Equilibrium Flip Ebook Pages 1 50 Anyflip Anyflip




Chemical Equilibrium Brilliant Math Science Wiki




Equilibrium Notes Chem1100 Chemistry 1 Studocu




For Homogeneous Equilibrium Why Are Liquids And Solids Included In The Equilibrium Constant When They Aren T In Heterogeneous Equilibria Chemistry Stack Exchange




Ppt Heterogeneous Equilibria Powerpoint Presentation Free Download Id
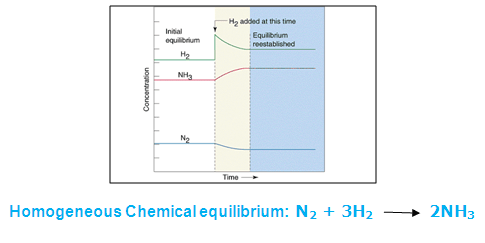



Eage Tutor




Solved Does The Following Equation Represent A Homogeneous Equilibrium Or A Heterogeneous Equilibrium Explain Your An




Equilibrium Part 1 Saitech Informatics
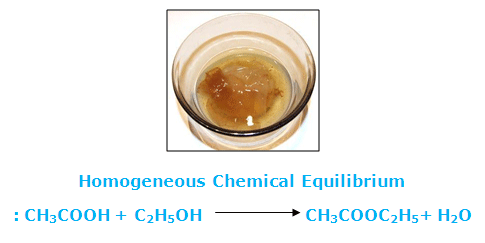



Eage Tutor




Difference Between Homogeneous And Heterogeneous Equilibrium Compare The Difference Between Similar Terms




2rm Chem 40 2nd Ans Check Pdf
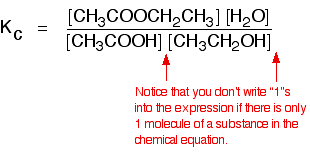



Kc Chemistry Libretexts



1



Equilibrium Constants Kp




1 Types Of Equilibrium And Le Chateliers Principle Mr Shieldsregents Chemistry U13 L02 Ppt Powerpoint




Question 19 2 Points Identify Each Of The Following As A Homogeneous Or Heterogeneous Eq Uilibrium Homeworklib



Www Utdallas Edu Son Chem1312 Chapter15a Pdf




Chemical Equilibrium What Is Equilibrium Expressions For Equilibrium



Exe




What Are Some Examples Of Homogeneous Reactions Quora



Real Life Applications Chemical Equilibrium Homogeneous And Heterogeneous Equilibria The Equilibrium Constant




6 2 Equilibrium Constants Dissociation Chemistry Chemical Equilibrium




Heterogeneous Equilibrium Homgeneous Equilibrium Concept Chemistry Video By Brightstorm




Heterogeneous To Homogeneous Melting Transition Visualized With Ultrafast Electron Diffraction Science



Chapter12




Compare Homogeneous And Heterogeneous Equilibria Chegg Com



Http Www Celinaschools Org Downloads Notes Equil D S Pdf



Synthesis Of A Molecularly Defined Single Active Site Heterogeneous Catalyst For Selective Oxidation Of N Heterocycles Nature Communications




Ppt Homogeneous Equilibria Powerpoint Presentation Free Download Id




1 10 Equilibrium Constant Kc For Homogeneous Systems Equilibrium Secondary Science 4 All




Table 1 From An Investigation Of Access Chemistry Lecturers Practice Of Pedagogic Content Knowledge In Chemical Equilibrium In An Access Programme Semantic Scholar




Q2 Differentiate Between Homog Lido




Which Of The Systems Described Below Give Homogeneous Chegg Com




Eqilibrium Principles Of Chemistry Ii Lecture Slides Docsity



Chemistry Equilibrium Part 10 Heterogeneous Homogeneous Equilibrium Cbse Class 11 Xi Video Dailymotion




What Is The Difference Between Homogeneous And Heterogeneous Equilibrium Slide Share




Answered I Directions Write The Equilibrium Bartleby




Homogeneous Vs Heterogeneous Equilibrium Reactions Study Com




Difference Between Homogeneous And Heterogeneous Equilibrium Compare The Difference Between Similar Terms




What Is The Trick To Know Whether The Reaction Is Heterogeneous Or Homogeneous Chemistry Equilibrium Meritnation Com



Www Unf Edu Michael Lufaso Chem46h 46chapter15 Pdf



Www Unf Edu Michael Lufaso Chem46h 46chapter15 Pdf




Section 15 3 Equilibrium Expressions Review Chegg Com




Real Life Applications Chemical Equilibrium Homogeneous And Heterogeneous Equilibria The Equilibrium Constant Life Application Real Life Chemical




Chemical Equilibrium Concept Of Dynamic Equilibrium Heterogeneous Equilibrium Equilibrium Constant Applications Le Chatelier S Principle Temperaturereactants Productspressure Volume Ppt Powerpoint



3




Homogeneous Equilibrium Model For Geomechanical Multi Material Flow With Compressible Constituents Sciencedirect



Heterogeneous Equilibria Qs Study



13 2 Equilibrium Constants Chemistry




1 Which Of The Following Is Equal For The Forward And Reverse




Next Page Page 19 Of 44 Question 19 6 Points Chegg Com
コメント
コメントを投稿